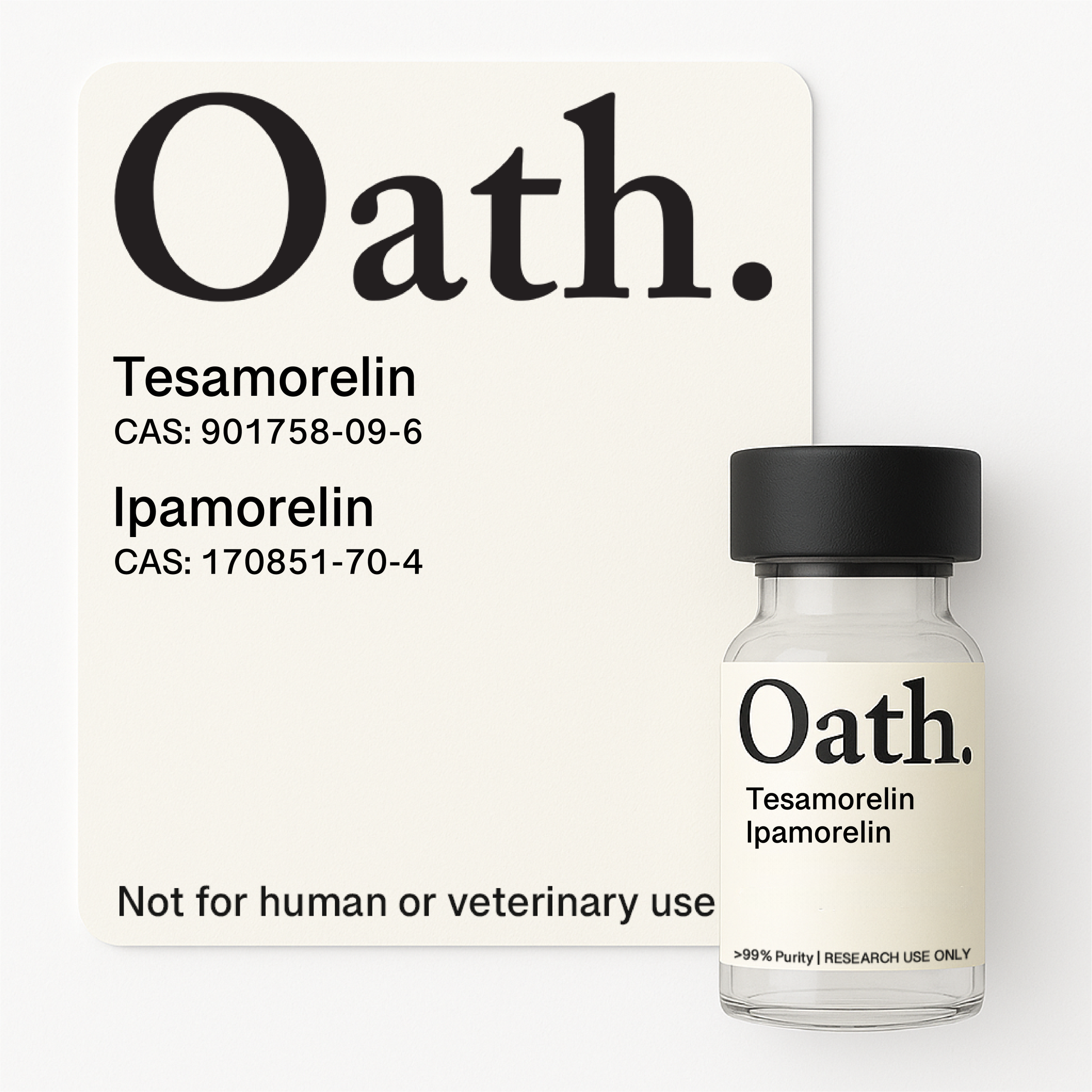
This synergistic blend combines two complementary growth hormone-releasing peptides studied for their combined effects on GH secretion and metabolic health. Tesamorelin, a GHRH analog, is researched for its ability to reduce visceral fat and increase IGF-1 levels, while Ipamorelin, a selective GHRP, offers the advantage of not elevating cortisol or prolactin like other GHRPs—designed for studies exploring growth hormone physiology and body composition.
Available in two concentrations: a standard 8mg blend (Tesamorelin 6mg + Ipamorelin 2mg) and a higher-dose 15mg option (Tesamorelin 10mg + Ipamorelin 5mg) for research requiring increased peptide loads.
2 Day FAST Shipping
99%+ Purity Tests
Ships from Gilbert Arizona
USA Lab Tested
Free bacteriostatic water with every order.